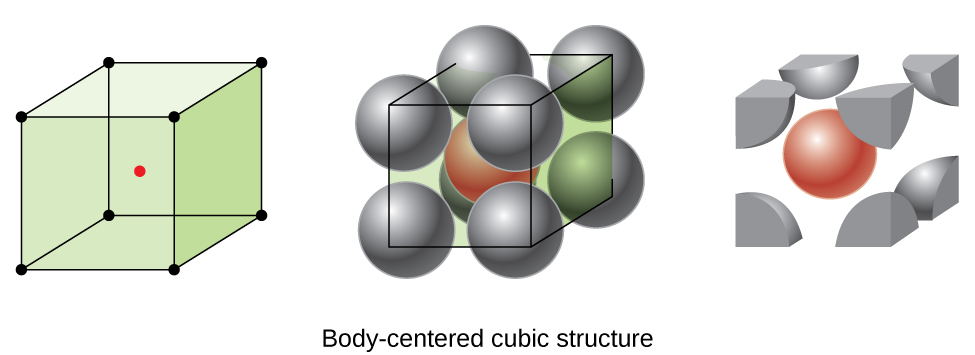
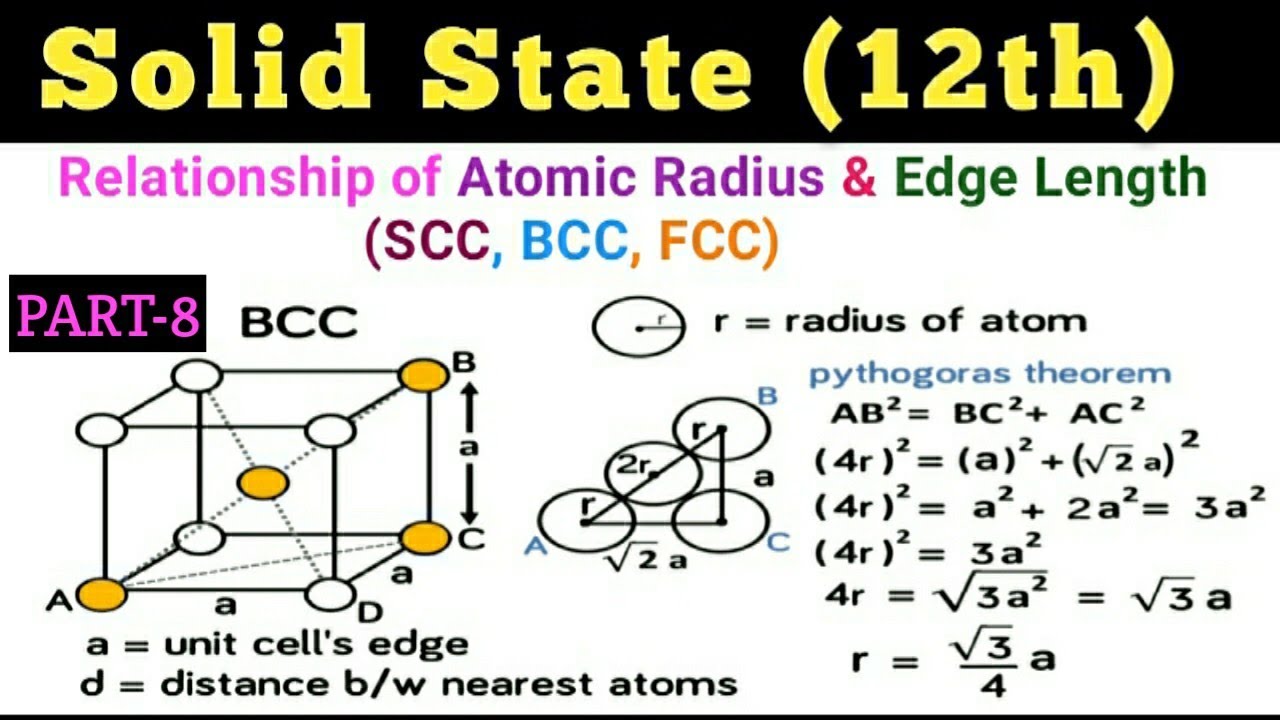
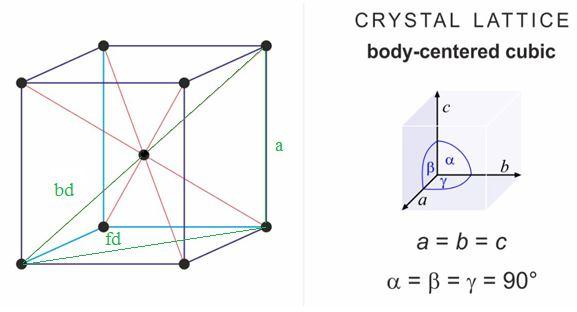
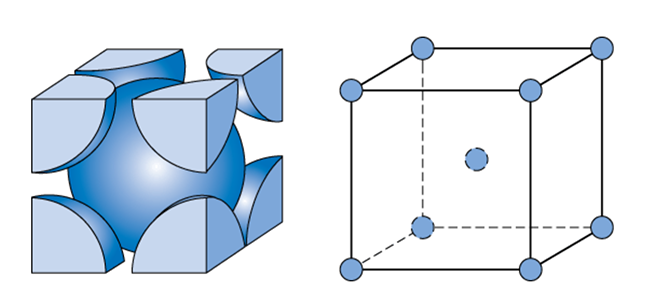
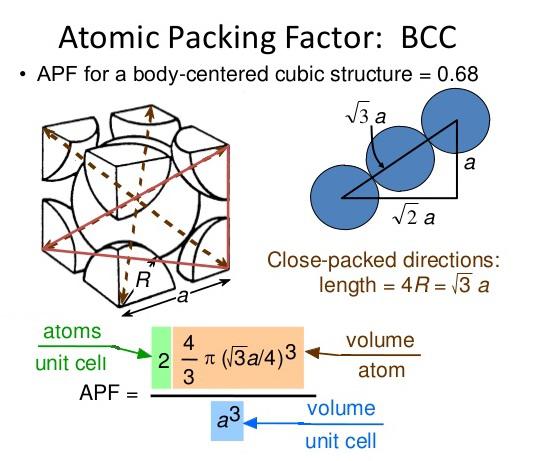
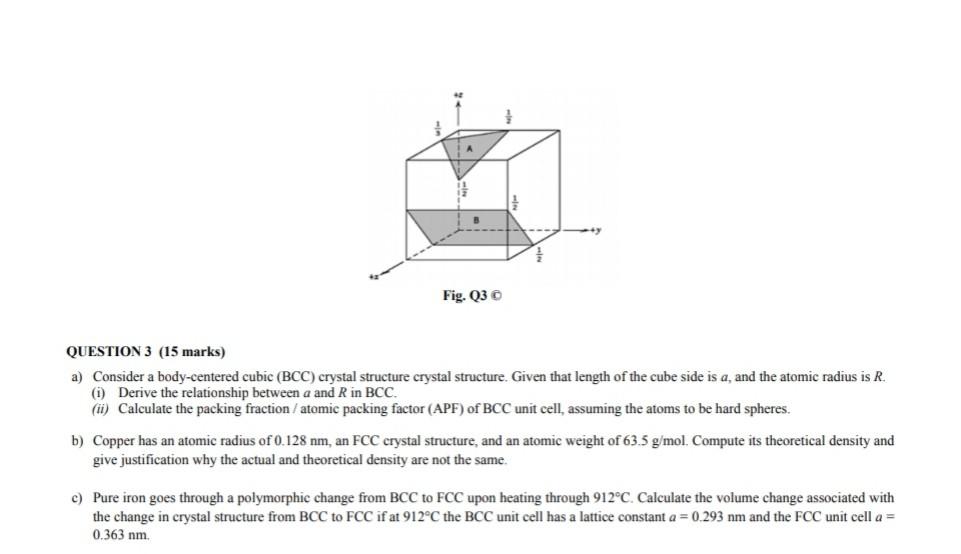
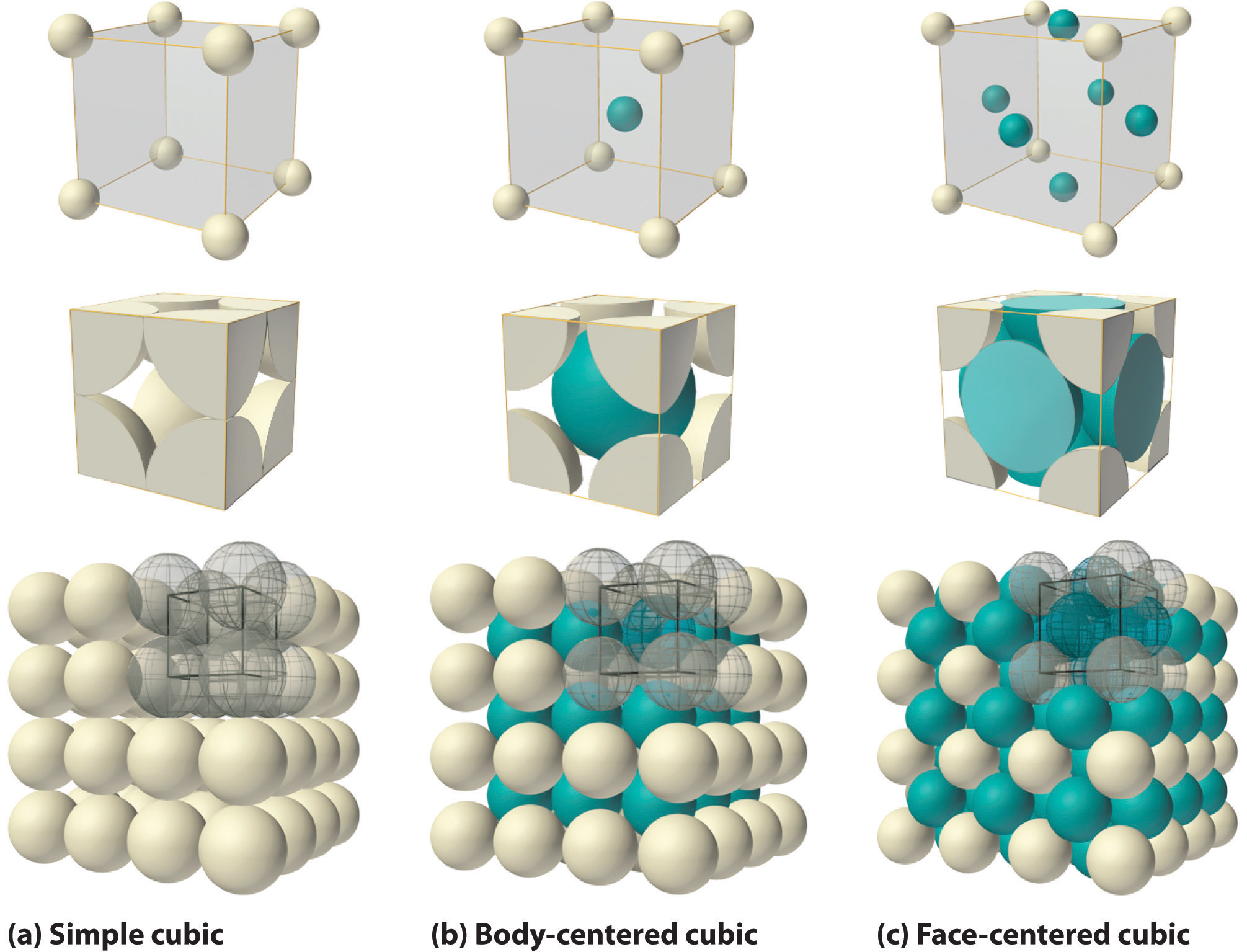
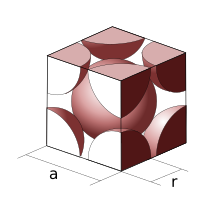
Lithium metal crystallises in a body centred cubic crystal. If the length of the side of the unit cell of lithium is 351 pm, the atomic radius of the lithium will be?
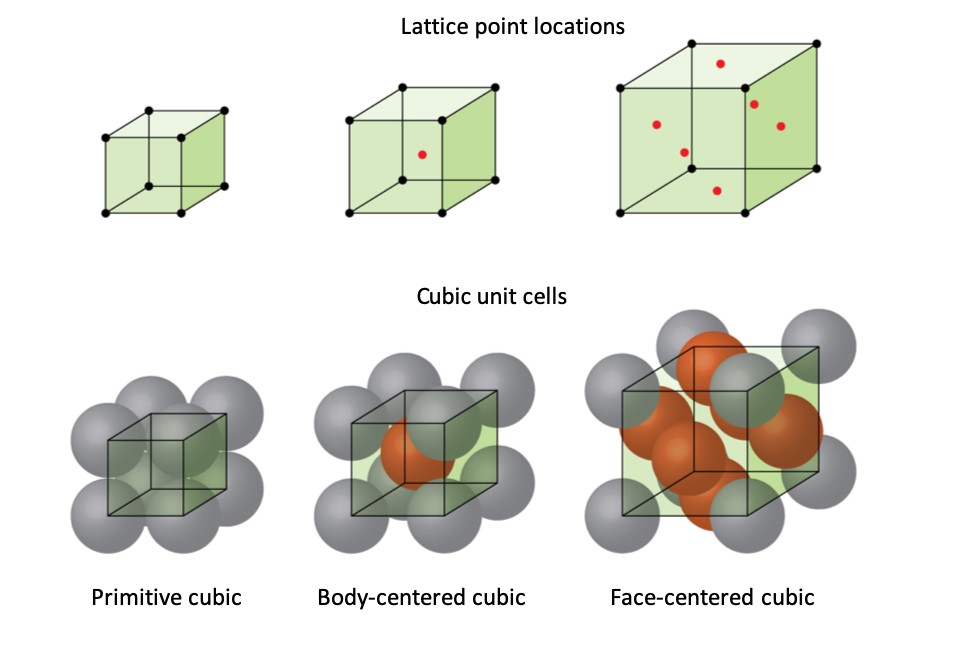
Types of Unit Cells: Body-Centered Cubic and Face-Centered Cubic (M11Q5) – UW-Madison Chemistry 103/104 Resource Book

Unit Cell Chemistry Simple Cubic, Body Centered Cubic, Face Centered Cubic Crystal Lattice Structu - YouTube
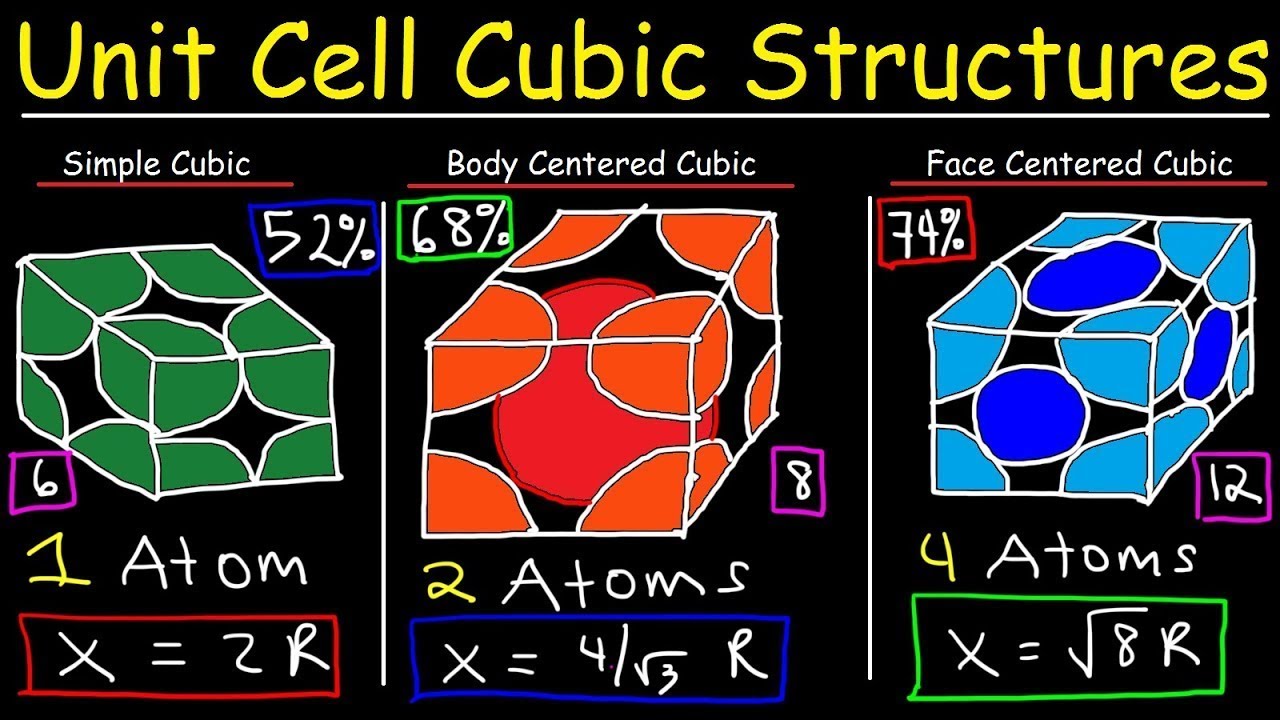
Unit Cell Chemistry Simple Cubic, Body Centered Cubic, Face Centered Cubic Crystal Lattice Structu - YouTube
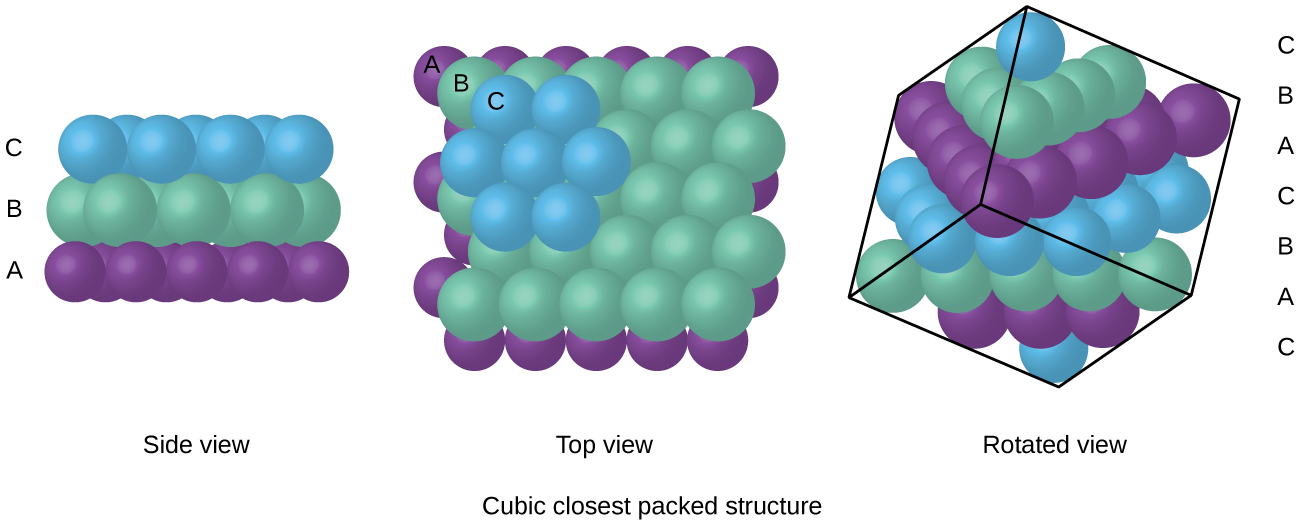
Types of Unit Cells: Body-Centered Cubic and Face-Centered Cubic (M11Q5) – UW-Madison Chemistry 103/104 Resource Book